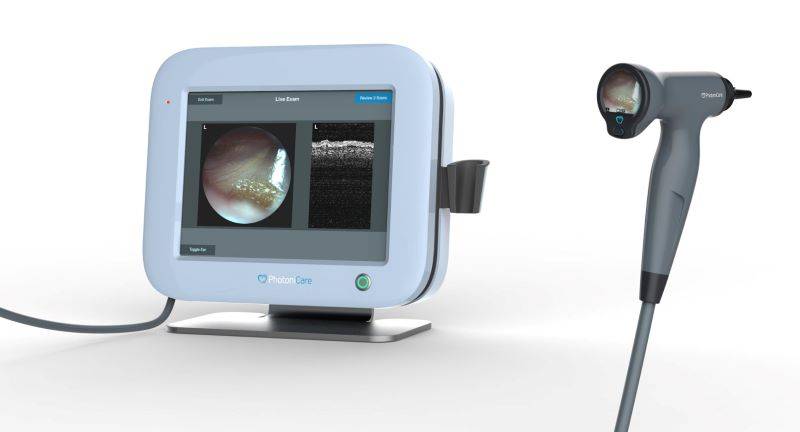
PhotoniCare is a local medical device startup that began in the bioengineering lab of the University of Illinois. They have specifically been working on technology that would help physicians in diagnosing and treating middle ear infections in children. That technology, TOMi scope, has received FDA clearance. It is designed to provide non-invasive imaging of the middle ear. From the announcement:
According to clinical guidelines, the presence of fluid in the middle ear is a primary indicator for determining infection. Designed to eliminate subjectivity and speculation, TOMi Scope allows physicians, for the first time, to directly visualize fluid in the middle ear, where ear infections reside, and to measure the fluid’s density, even in the presence of wax – providing objective data upon which to base their decisions.
If you’ve had little kids, especially those prone to ear infections, this is pretty huge news.
They will be having an open house at their new location, 1902 Fox Dr. Suite F in Champaign, on February 13th from 5 to 7 p.m., to celebrate the launch of the device.
Top image: A screen with a white frame that shows images of the inside of the ear. To its right is a gray scope with a black tip that is connected to the screen. Image from PhotoniCare Facebook page.